Nirmatrelvir is an antiviral medication developed by Pfizer for treating mild to moderate cases of Covid-19. It is sold under the brand name Paxlovid.
The medication, which is taken orally, contains two medications: “Nirmatrelvir helps stop the SARS-CoV-2 virus from duplicating itself within the body. And ritonavir – a drug originally approved by the FDA for the treatment of HIV infection in adults and children – slows down the body’s breaking down of nirmatrelvir, allowing it to stay in the body longer” (Pelc).
In December 2021 the Food And Drug Administration (FDA) announced that Paxlovid had been granted Emergency Use Authorization. Eligibility requirements are as follows: “For the treatment of mild-to moderate COVID-19 in adults and children [12 years of age and older weighing at least 88 pounds (40 kg)] with a positive test for the virus that causes COVID-19, and who are at high risk for progression to severe COVID-19, including hospitalization or death, under an EUA.” For more information on the medication, including possible side-effects, visit here.
Some patients reported a nasty metallic taste in their mouth after taking the medication that can last for a while. This condition is called dysgeusia. Despite this unpleasant side effect, medical experts strongly suggest continuing with the full course of the treatment (Pelc).
Social Media Trends as of September 11, 2022
Facebook #paxlovid: 7,000 people are posting about this
Instagram #paxlovid: 4,384 posts
TikTok #paxlovid: 16.7 million views
YouTube #paxlovid: 604 videos and 350 channels
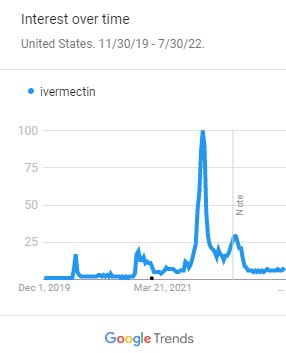
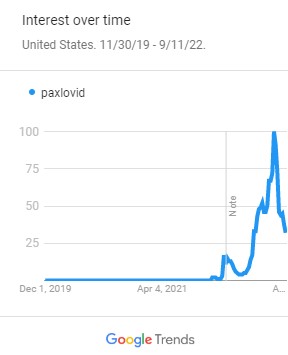
Google Trends: paxlovid first appeared during the week of October 31, 2021 when news of the antiviral medication being used as a possible cure for the treatment of Covid-19 started to appear. The Omicron variant was also at its peak around this time. The popularity of Paxlovid as a search term reached its peak during the week of July 17, 2022 when the medication became more widely available.
Sources:
“EMERGENCY USE AUTHORIZATION (EUA) OF PAXLOVID.” Food and Drug Administration. August 26, 2022. URL: https://www.fda.gov/media/155051/download.
Pelc, Corrie. “Paxlovid mouth: What is it and how to get rid of it.” Medical News Today. August 23, 2022. URL: https://www.medicalnewstoday.com/articles/paxlovid-mouth-what-is-it-and-how-to-get-rid-of-it.